|
|
|
Extra Credit!! Answer the one you DID NOT answer on the
exam. Bring it in on Monday!
Honor system….you must complete this on your own.
3) Explain each of the following in terms of atomic and molecular structures and/or intermolecular forces.
(a) Solid K conducts an electric current, whereas solid KNO3 does not.
(b) SbCl3 has a measurable dipole moment, whereas SbCl5 does not.
(c) The normal boiling point of CCl4 is 77 oC, whereas that of CBr4 is 190 oC.
(d) NaI(s) is very soluble in water whereas I2(s) has a solubility of only 0.03 gram per 100 grams of water.
4)
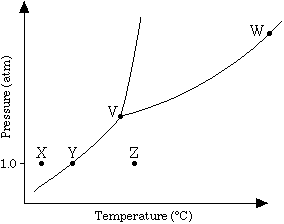
The phase diagram for a pure substance is shown above. Use this diagram and your knowledge about changes of phase to answer the following questions.
(a) What does point V represent? What characteristics are specific to the system only at point V?
(b) What does each point on the curve between V and W represent?
(c) Describe the changes that the system undergoes as the temperature slowly increases from X to Y to Z at 1.0 atmosphere.
(d) In a solid-liquid mixture of this substance, will the solid float or sink? Explain.